- Characterized by glomerular scarring from podocyte injury and depletion4
- Patients typically present with subnephrotic to nephrotic ranges of proteinuria, hypertension, microscopic hematuria, and renal insufficiency
- Approximately 20-25% of adult patients undergoing biopsy evaluation for idiopathic glomerulonephritis (GN) are diagnosed with primary (idiopathic) FSGS
- Approximately 35% of adult patients undergoing biopsy evaluation for idiopathic nephrotic syndrome are diagnosed with FSGS, making it the most common lesion found5
- The remaining patients have secondary FSGS from genetic factors, drugs, infection, or other structural/functional adaptations
- Most common primary glomerular disease leading to ESRD
- Incidence rate estimated to be about 2.7 patients/million on average in the United States
-
Higher male dominance
- 1.5:1 male-to-female ratio
- Can occur at any age
- Most common in African Americans, followed by Hispanics and Caucasians4
-
Prognosis varies depending on levels of proteinuria:
- Non-nephrotic levels — <15% progress to ESRD over 10 years
- Nephrotic levels — ≤50% progress to ESRD over 5-10 years
- Massive levels — ESRD within 2-3 years5
-
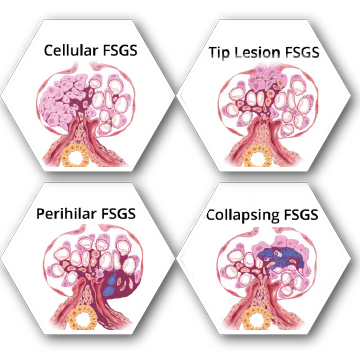
Histologically, FSGS can be observed by 5 types of scarring patterns:
- Cellular variant — overabundance of cells within the glomerulus, impinging on the blood vessels
- Tip lesion variant — scarring appears at the tip or pole of the glomerulus (best overall outcomes)
- Perihilar variant — scarring appears where blood vessels enter and exit the glomerulus
- Collapsing variant — scarring affects the entire glomerulus leading to a collapsed appearance (lowest overall outcomes)
- Not otherwise specified (NOS) variant – must exclude other variants; defined by blocked glomerular capillaries form extracellular matrix accumulations6
ESRD: end-stage renal disease
Recommendations are based on the KDIGO Clinical
Practice Guideline for Glomerulonephritis
- Treatment aimed towards remission of proteinuria to slow/control progression of ESRD
- Prognosis goals: proteinuria reduction, complete or partial remission of nephrotic syndrome, and stable or improved renal function
- Options are based on degree and persistence of nephrotic-range proteinuria and kidney function
- Initial therapy upon diagnosis should consist of daily or alternating-day doses of corticosteroids if FSGS presents with nephrotic syndrome
- 4-16 weeks of treatment until remission
- CNIs are prescribed for first line of defense if patients cannot tolerate/are resistant to corticosteroids or have contraindications
- Cyclosporine is recommended, but tacrolimus is mentioned as a potential option for intolerant patients
- If a patient is resistant to corticosteroids and intolerant to CNIs, a combination therapy of MMF and high-dose dexamethasone is recommended
- As of 2012, the KDIGO guidelines list the following therapies as potential treatment options, but they are not recommended this agent:
- Alkylating agents (cyclophosphamide/chlorambucil)
- Monoclonal antibodies (rituximab)10
Alternative option not listed in 2012 KDIGO Guidelines
- The product is an FDA approved treatment option11
CNI: calcineurin inhibitor; ESRD: end-stage renal disease; MMF: mycophenolate mofetil.
Treatment Options
|
|
|---|---|
 Corticosteroids10 Corticosteroids10
|
 Cytotoxic Agents10 Cytotoxic Agents10
|
 Monoclonal Antibodies10 Monoclonal Antibodies10
|
 Calcineurin Inhibitors10 Calcineurin Inhibitors10
|
 Immunosuppressive Agents10 Immunosuppressive Agents10
|
 The Product®11* The Product®11*
|
*FDA approved, but not enough contemporaneous data for KDIGO to make a use recommendation