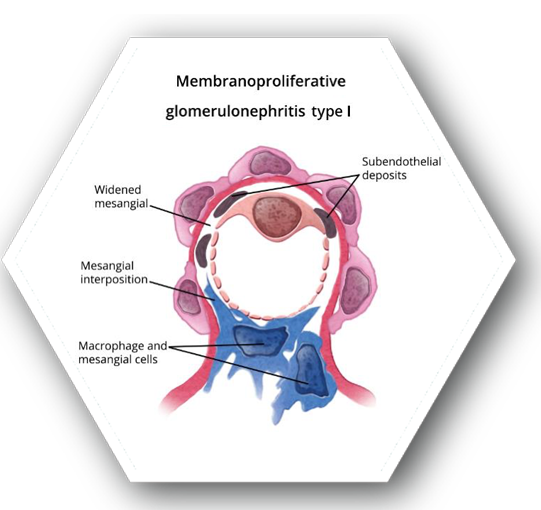
- Characterized by diffuse mesangial cell proliferation, endocapillary proliferation, and thickening of glomerular basement membrane9
-
MPGN was previously classified into 3 categories based on histological findings (Types I, II, and III). Presently, a new classification system has been developed based on pathophysiology:
- Immune complex-mediated MPGN – defined by deposition of immune complexes within the glomeruli, leading to an influx of inflammatory cells in the mesangium and capillary loops (formerly classified mostly type I)6
-
Complement-mediated MPGN – defined by deposition of complement products in the mesangium and capillary walls
- Dense deposit disease (DDD) - MPGN with intramembranous dense deposits (formerly classified type II)
- Component 3 glomerulonephritis (C3GN) without dense deposits (formerly classified mostly type III)9
- With varying forms of MPGN, patients may present with microhematuria and non-nephrotic proteinuria, nephrotic syndrome with mild renal insufficiency, clinically progressive GN, or as nephritic syndrome with proteinuria and red blood cell casts
- Typically idiopathic for children, but often secondary to cryoglobulinemia and Hepatitis C infection in adults
- Causes <5% of all primary GN in North America and Europe
- Accounts for 5-10% of causes of nephrotic syndrome in adults and children
- No gender dominance
- Most commonly found in Caucasians in the United States
-
Prognosis is poor for children and adults
- Children: 40-50% progress to ESRD after 10 years
- Adults: 50% die or need renal replacement therapy6
ESRD: end-stage renal disease; GN: glomerulonephritis.
Recommendations are based on the KDIGO Clinical
Practice Guideline for Glomerulonephritis
-
Treatment aimed towards reducing proteinuria to slow progression of ESRD only for patients with idiopathic MPGN
- Secondary MPGN should be directly treated for the underlying cause
- Prognosis goals: prevention of ESRD
- Options are based on progression of renal failure
-
Initial therapy upon diagnosis should consist of daily or alternating-day doses of corticosteroids combined with cyclophosphamide or MMF
- Limit treatment to less than 6 months10
Alternative options not listed in 2012 KDIGO Guidelines
-
Idiopathic MPGN
- CNIs may be used if patients cannot tolerate corticosteroids
- Rituximab is suggested if treatment with cyclophosphamide is unsuccessful
-
C3 glomerulonephritis
- No trials have been evaluated for therapy options12
-
Dense deposit disease
- Immunosuppressive therapies are unsuccessful
- Pulse steroids (methylprednisone) may be used for patients with rapidly progressive glomerulonephritis13
- The product is an FDA approved treatment option11
CNI: calcineurin inhibitor; ESRD: end-stage renal disease; MMF: mycophenolate mofetil
Treatment Options
|
|
|---|---|
 Corticosteroids10,13 Corticosteroids10,13
|
 Cytotoxic Agents10 Cytotoxic Agents10
|
 Calcineurin Inhibitors12 Calcineurin Inhibitors12
|
 Immunosuppressive Agents10 Immunosuppressive Agents10
|
 Monoclonal Antibodies12 Monoclonal Antibodies12
|
 Acthar® Gel11* Acthar® Gel11*
|
*FDA approved, but not enough contemporaneous data for KDIGO to make a use recommendation