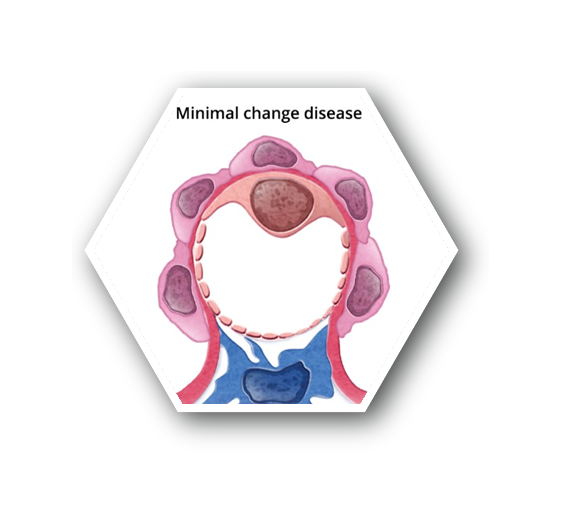
- Characterized by fusion of podocyte foot processes and minimal glomerulus alterations, depicted as microscopic lipid droplets while observed under light microscopy8
-
Patients typically present with edema and nephrotic syndrome with accompanying acellular urinary casts7
- Fluid retention often exceeds 3% of body weight6
- Pathogenesis for idiopathic MCD is unknown – believed to be a consequence of cytokines that alter podocyte integrity and increase permeability to protein7
- Secondary MCD may result from malignancies, drugs, or use of interferons
- Causes nephrotic syndrome in about 90% of children <10 years of age, about 50-70% of older children, and 10-15% of adults
- Incidence rate estimated to be about 2-7 children/100,000 on average worldwide
-
Higher male dominance
- 2:1 male-to-female ratio
- Most common in children ages 2-7
- Less common in adults
- Typically found in South Asians and Native Americans
-
Prognosis is usually good since MCD does not progress to renal failure6
- However, relapses recur in 70-75% of children after first remission7
Recommendations based on the 2012 KDIGO
Clinical Practice Guideline for Glomerulonephritis
-
Treatment aimed towards remission of proteinuria
- Prognosis goals: complete remission of nephrotic syndrome
- Options are based on steroid-dependency and frequency of relapse
-
Initial therapy upon diagnosis should consist of daily or alternating-day doses of corticosteroids
- 4-16 weeks of treatment until remission
- Cyclophosphamide is first recommended for frequently relapsing patients that cannot tolerate high-dose corticosteroids or have contraindications
-
CNIs are prescribed if patients relapse despite cyclophosphamide or wish to preserve fertility
- Options include cyclosporine and tacrolimus
- MMF therapy is recommended for patients intolerant to corticosteroids, cyclophosphamide, and CNIs10
Alternative options not listed in 2012 KDIGO Guidelines
- Chlorambucil provides similar efficacy to cyclophosphamide, but is not a preferable recommendation due to higher frequency of adverse effects
- Rituximab studies have shown favorable results in significantly reducing relapse for patients unresponsive to previous treatment options6
- The product is an FDA approved treatment option11
CNI: calcineurin inhibitor; MMF: mycophenolate mofetil.
Treatment Options
|
|
|---|---|
 Corticosteroids10 Corticosteroids10
|
 Cytotoxic Agents6,10 Cytotoxic Agents6,10
|
 Calcineurin Inhibitors10 Calcineurin Inhibitors10
|
 Immunosuppressive Agents10 Immunosuppressive Agents10
|
 Monoclonal Antibodies6 Monoclonal Antibodies6
|
 Acthar® Gel11* Acthar® Gel11*
|
*FDA approved, but not enough contemporaneous data for KDIGO to make a use recommendation