- Recurrence of glomerulonephritis as an underlying cause of ESRD in kidney transplants can range from 30-50% worldwide (~30% in the US)
- A wide array of variables affect recurrence, which can greatly impact kidney graft survival. Some of these variables include:
- Pre-transplant disease (higher risk with FSGS and MPGN)
- Recurrence of disease post-transplant
- Time of recurrence
- To help prevent graft failures, transplant patients often have their kidney biopsied to detect GN recurrence as early as possible. These biopsies typically occur at discharge, after 3 weeks, at 3-6 months, at 1 year, and after 3 years25
(click for more information)
X
Recurrent Membranous Nephropathy26
- 40-50% recurrence for primary/idiopathic MN overall
- 60-76% in patients with anti-PLA2R antibodies pre-transplant
- 28-30% in patients without anti-PLA2R antibodies pre-transplant
- Measuring anti-PLA2R antibodies throughout transplant process may help assess for risk of recurrence and/or progression in patients with history of primary MN
- Discovered mostly within first year of transplant, but still at high risk at 5 years
- Most physicians begin treatment when proteinuria levels are progressive and reach 1000 mg/day
- Rituximab treatment 4-6 months before transplant may reduce anti-PLA2R antibodies and prevent recurrence
- Death-censored graft loss in 45% of patients
- No FDA-approved nephrology treatment options
- No treatment recommendations in the 2009 KDIGO Transplant Recipient guidelines27
- Alternative treatment options not listed in the 2009 KDIGO guidelines: *26
- Calcineurin inhibitors (cyclosporine or tacrolimus)
- Corticosteroid/alkylating agent (cyclophosphamide or chlorambucil) combination therapy
- May cause severe leukopenia – recommend stopping MMF portion of transplant immunosuppression therapy
- Rituximab
- Typically safer, better tolerated, and does not involve modifying transplant immunosuppression – potential recommendation for using as primary therapy
- Used regardless of anti-PLA2R antibody status
- Can lead to 80% of patients seeing complete or partial remission
X
Recurrent Focal Segmental Glomerulosclerosis28
- 20-50% recurrence for primary FSGS, but may exceed 80% with prior history of graft loss
- Can recur immediately or many years later
- Difficult to diagnose later on due to secondary factors (i.e. renal mass, injury, drugs), common in post-transplantation
- 57% graft loss by 5 years
- Evidence is inconclusive if treatment with plasmapheresis (PE) or rituximab before transplant may prevent FSGS recurrence
- If urine protein creatinine ratio exceeds 1 g/g, renal biopsy is performed
- No FDA-approved nephrology treatment options
- Treatment recommendations from the 2009 KDIGO Transplant Recipient guidelines:27
- Plasmapheresis
- Cyclosporine (alone or in combination with PE)
- Angiotensin-converting enzyme (ACE) inhibitor and/or an angiotensin II receptor blocker (ARB)
- Alternative treatment options not listed in the 2009 KDIGO guidelines:*28
- Plasmapheresis (alone or in combination with rituximab)
- Rituximab
- Corticosteroid/calcineurin inhibitor combination therapy
- If no response, ACTH (40 units 2x/week – increase to 80 units 2x/week if tolerated)
- 50% of 20 recurrent or de novo patients in recent retrospective study achieved partial or complete remission with ACTH therapy
- Limitations: retrospective study with no control group. Not all patients were treated similarly; this included some subjects who received initial prophylactic TPE or rituximab as a preventative measure at their treatment center. A variety of factors may have influenced the improvement in proteinuria, including TPE given with The product
- Safety:
- 8 patients had allograft failure during the follow-up period. 5 graft failures were attributed to recurrent or de novo FSGS despite the use of The product, 1 was complicated by cytomegalovirus disease, and 1 by JC nephropathy
- 1 patient died due to an aortic dissection while on therapy. 2 patients died during the post-therapy follow-up period
X
Recurrent IgA Nephropathy25,29
- 30% variable recurrence rate
- Typically manifests after 5 years post-transplant
- Patients tend to have better graft survival with IgAN versus other GN types
- Higher risk of losing graft if recurrent
- If patients present with new microscopic hematuria, new or worsening proteinuria, or an increase in creatinine, a renal biopsy is performed to confirm diagnosis
- Patients did not receive any therapeutic benefits from altering immunosuppressive therapy during treatment
- No FDA-approved nephrology treatment options
- Treatment recommendations from the 2009 KDIGO Transplant Recipient guidelines:27
- Angiotensin-converting enzyme (ACE) inhibitor and/or an angiotensin II receptor blocker (ARB)
- Antithymocyte globulin induction
- Alternative treatment options not listed in the 2009 KDIGO guidelines:*25
- Corticosteroids
X
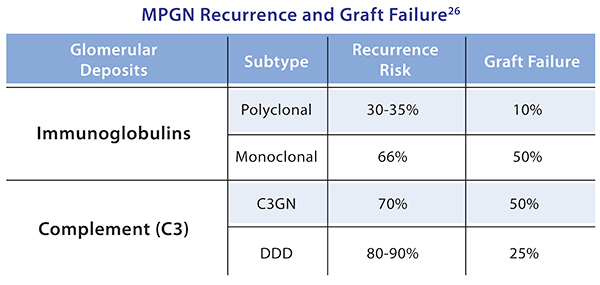
Recurrent Membranoproliferative Glomerulonephritis26,29
- Recurrence and graft failure rates are dependent on the MPGN classification
- Increased risk of recurrence associated with low complement levels and living- related kidney transplantation
- Diagnosis and classification of disease performed through renal biopsy
- No FDA-approved nephrology treatment options
- Treatment recommendations from the 2009 KDIGO Transplant Recipient guidelines:27
- Plasmapheresis
- Cyclosporine
- Cyclophosphamide
- Alternative treatment options not listed in the 2009 KDIGO guidelines:*25
- Rituximab
- Eculizumab